
More and more consumers ask if sunscreens are safe. This question boils down to safety assessment as the European Union requires it for all Cosmetic Formulations. But it goes beyond safety of risk assessment. The user is finally also involved and then we rather talk about risk management, e.g. as Diffey explained it in his book: Sun Protection book – a risk management approach” [1].
Risk Management is the process leading to a choice among alternative courses of action and establishing the priorities and strategies for implementation. The process of risk management can be broadly split into determination and evaluation, i.e. a scientific activity and a social activity (Figure 1).
People perceive risk differently depending on their awareness and understanding of the risk in question, and also on their per- ception of how they might suffer personally as a consequence of the risk. Diffey was discussing solely the risk of UV-exposure, but with the discussions about the efficacy and safety of sunscreens, a new dimension is added to the risk management. Sunscreen, the very means intended to reduce the risk of UV radiation has now become itself the subject of risk management.
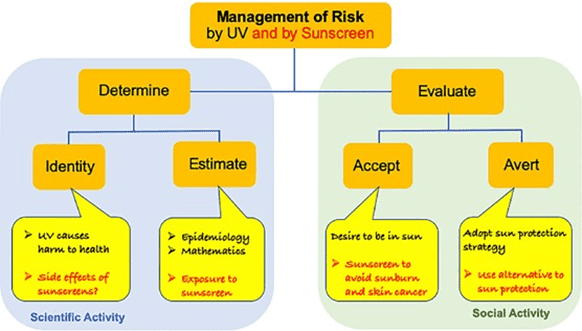

Figure 2
Risk = Hazard x Exposure
(Tiger = hazard, Cage prevents exposure) The problem with this example:
it is too simplistic and does not consider the subtleties of many hazards. For other hazards, awareness or knowledge of them may not be shared or even known by anyone.
The tiger in the cage – when is a hazard not a hazard?
Standard health and safety courses often stress the distinction between hazard and risk. This distinction can be lost on mere mortals (students get confused too) and, in an attempt to help the matter, we are told the apparently simple definition of a hazard as “something with the potential to cause harm”; we are told “something” can mean “anything” – object, chemical, activity, condition … Despite the apparent elegance of this definition, it is recursive and woolly in practice. We are then sometimes told that hazards are immutable (that is hazards don’t change) but risks do. This, of course is not quite the truth. Closer to the truth is what we recognize as a significant hazard, changes [2].
Chemical substances are a classic example of this – especially the carcinogens. But knowledge is a wonderful thing and there is power in hindsight. The Curies probably didn’t recognize pitchblende as quite so hazardous (radioactive); the “wonder material” that is asbestos hasn’t always been connected with cancer either. On the other hand, what we breath has 80% nitrogen, practically, a ‘hazard’ might not be one thing or one property but a combination of things which, if occurring together, presents a hazard (a potential for harm) [2].
Science moves on and it is only by examining and testing substances (or having accidents/incidents) that we become aware. This has led to what is called the “precautionary principle” in chemical/biological safety – i.e. treat it as dangerous unless proved otherwise (though we would not recommend widespread use of this principle as it can lead to extreme risk aversion and impeding innovation).
Hazard for Humans and Safety Assessment
Percutaneous absorption is the biggest issue of sunscreen safety. The soluble organic UV filters all pass the skin to some extent. Important parameters of prediction are the molecular weight (< 500 DA) and the Water/Octanol partitioning coefficient (-1 < log Po/w < 4). Data provided in [3] (table 2). The highest skin per- meation by BP3 (oxybenzone) does not come as a surprise, with its molecular weight of 228 Da and a partition coefficient of
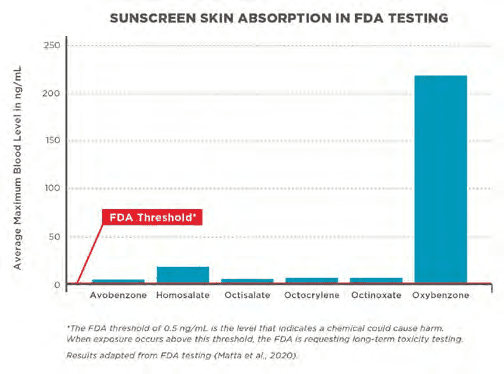
could harm. When exposure occurs above this threshold, the FDA is requesting
long-term toxicity testing [5].
LogPo/w 3.5 (Figure 3). The inorganic mineral UV Filter particles (nano) cannot pass the skin and are considered GRASE by the FDA [4].
All six of the tested active ingredients administered in four different sunscreen formulations were systemically absorbed and had plasma concentrations that surpassed the FDA threshold for potentially waiving some of the additional safety studies for sunscreens.
These findings do not indicate that individuals should refrain from the use of sunscreen, according to a recommendation of the FDA [4,5].
Discussion
The systemic availability of some UV filters has been known for some time. The question arises: What does systemic availability mean? Opinions range from “continuing to use sunscreen” to “banning it completely”. Given that the purpose of applying sunscreens is to prevent damage, the latter is not justified as long as
there is no evidence that these UV filters are harmful. Further studies are necessary and are required by the FDA. In the meantime, the available data on UV filters can be used to estimate their percutaneous absorption. The safety aspect is not only a question of chemical properties (hazard), but also of exposure. It is therefore
not a solution to denounce UV filters only because of their hazard potential. For UV filters to have a very low percutaneous absorption, two parameters are crucial: molecular weight and polarity, expressed as the oil/water partition coefficient. UV filters with inherently very low or no percutaneous absorption are particulate
UV filters, both inorganic and organic. However, such an assessment is only an indication and does not replace a detailed risk assessment as defined, for example, in European legislation.

five minutes to ruin it. If you think about that,
you’ll do things differently”
Warren Buffet, Investor
Conclusion/Trust (Consumer Confidence)
Everybody agrees that what is common to Sunscreens and Drugs is that they have to be SAFE and EFFECTIVE. These two conditions of the sunscreen are however not sufficient to assure good sun protection. Good sun protection can only happen when the user of the sunscreen fulfills his or her part of the equation. That is where trust plays an important role. If somebody does not believe in the efficacy and/or the safety of sunscreen, chances that he or she will apply it frequently are low. If, in addition applying of sunscreen feels unpleasant and is complicated, achieving good
sun protection becomes even less likely.
What could change this vicious cycle is trust, i.e. consumer confidence in the efficacy and the safety of sunscreen. Trust can be built by facts, science and transparency. False or exaggerated claims can destroy trust much faster than it can be built. Next, we will discuss nano size UV filters and why “nano” means “BIG”.
References:
1 Diffey B, Sun Protection: A Risk Management Approach., IOP Publishing Ltd, Institute of Physics Publishing, Bristol, UK (2017).
2 David Towlson is director of training and quality at RRC International (2015) https://www.shponline.co.uk/common-workplace-hazards/tiger-cage-hazard-not-hazard/ accessed 2020-09-26
3 Hanay C, Osterwalder U. Challenges in Formulating Sunscreen Products. Curr Probl Dermatol.
2021;55:93-111. doi: 10.1159/000517655. Epub 2021 Oct 25. PMID: 34698033.
4 Matta MK, Zusterzeel R, Pilli NR, et al. Effect of Sunscreen Application Under Maximal Use Conditions on Plasma Concentration of Sunscreen Active Ingredients A Randomized Clinical Trial. JAMA.
doi:10.1001/jama.2019.5586.Published online May 6, 2019.
5 Matta MK, Florian J, Zusterzeel R, Pilli NR, Patel V, Volpe DA, Yang Y, Oh L, Bashaw E, Zineh I, Sanabria C, Kemp S, Godfrey A, Adah S, Coelho S, Wang J, Furlong LA, Ganley C, Michele T, Strauss DG.
Effect of Sunscreen Application on Plasma Concentration of Sunscreen Active Ingredients: A Randomized Clinical Trial. JAMA. 2020 Jan 21;323(3):256-267.

Uli Osterwalder
Uli Osterwalder studied Chemical Engineering at ETH Zurich, Switzerland and at the University of Houston in Houston, Texas. He joined Ciba-Geigy in Basel in 1979 where he first developed a Phosgene Generator in central process development. Later he developed his leadership skills in Project Management and Process Analytics. At Ciba Specialty Chemicals Uli Osterwalder helped establish new business development in Fabric Care and Personal Care. After the acquisition by BASF SE he became Senior Marketing Manager and Scientific Adviser in Sun Care in Ludwigshafen and Duesseldorf.
2016 he came back to Basel, working for DSM as senior Senior Scientific Adviser suncare for two years. 2018 he started his own company, Sun Protection Facilitator GmbH and is committed to contribute to further improvements in sun protection. Uli Osterwalder works for ISO on the development of new UV protection assessment methods and is now chairing the technical committee ISO TC/217 (Cosmetics). He is author and co-author of numerous scientific articles and book chapters on sun protection.



