MicroSilver BG
MicroSilver BGIntroduction and summary
Bacteria were on earth 4 billion years before humans (Lusk et al. 2019). They reproduce within a few minutes or hours, whereas humans need around 30 years for one generation. Within a week, bacteria can therefore go through as many generations as humans have since the beginning of the Egyptian Empire. This makes them extremely adaptable organisms that are often ahead of human evolution in many respects. Antibiotics have been invented as weapons by microorganisms to improve their competitiveness.
Therefore, bacteria handled antibiotics already when humans did not even know that antibiotics existed. Today, in the race with the pharmaceutical industry, they can always outwit its synthetic antibiotics with improved resistance within short time already after the introduction of new antibiotics.
Human evolution has been taking place also in tight interplay with microbial life forms around us, some of which now reside on our body where they form beneficial microbial communities.
They form what today is called the human microbiome. As early as 1938, microbes on the skin were divided into socalled resident skin flora and transient skin flora (Price 1938). The term “resident” germs is used to describe the individual “in-house” germs, while transient germs come from outside, i.e. from the environment, and settle on the human skin. In healthy skin, resident germs are found both on the skin surface and as a nursery version in deeper skin layers (Elsner 2003). From the deeper layers, the resident skin flora can regrow within a few hours (Hendley 1991, Marples 1974). The natural skin flora of a healthy person reproduces itself and is crucial for health and immunological defence.
There are numerous local microbiomes with different composition which can be found on the body (Costello et al. 2009). These local microbiomes are representing a natural flora of high diversity.
At the same time, they often show a surprising local stability. The natural skin microbiome is also crucial for maintaining an intact skin barrier and as a protection against infection. A healthy skin microbiome thus can deliver prophylaxis against a lot of inflammatory skin conditions which are increasingly in the focus of modern skincare especially for persons with stressed skin.
Bio-Gate has introduced MicroSilver BG™ as a natural, powerful skin conditioner with antimicrobial properties to protect the human skin against unwanted (“dysbiosis”) skin conditions where the healthy microbiome is known to get into a disarray. Here we prove that this 10μm porous microparticulate metallic silver powder does not damage the natural skin microbiome, but rather provides a supportive role to enhance modern advanced skincare products. The results show that MicroSilver BG™ does not actually affect the skin’s microbiome profile, making it a microbiomefriendly ingredient for modern skincare for stressed skin.
Microbiome
Human skin is always colonized by bacteria, viruses, and fungi. Skin, the largest exterior interface of the human body acts as a physical barrier to prevent the invasion of foreign outer pathogens. Some skin diseases are the result of unwanted damaged microbiomes. These dysbiosis, can be reversed or avoided by skincare which supports the healthy skin microbiome. Dysbiosis can be associated with microbiome alterations. They are caused by microorganisms, which can subsequently cause inflammation and further changes in the human immune response (De Pessemier 2021).
Currently, the market is generally focused on offering prebiotic and probiotic solutions. Consumers are likely to be familiar with probiotics with products designed to address specific concerns and benefits, such as reducing wrinkles, while others place the emphasis on nature, overall skin health, holistic lifestyles, and green beauty. These are skin care solutions which work by properly balancing the healthy microbes to preserve the microbiome.
MicroSilver BG™ – Microbiome friendly antimicrobial
MicroSilver BG™ has been used for almost two decades to care for stressed and irritated skin (Ekanayake-Mudiyanselage 2007, Müller-Steinmann 2008, Neub 2009, Müller-Steinmann 2011, Goldbach 2011, Barbareschi 2015, Steinrücke 2017a, Steinrücke 2017b). MicroSilver BG™ releases silver ions that bind to cysteine side chains in proteins and thus destroy their function. This unspecific mechanism, called the oligodynamic effect of silver, is the reason why silver also works against antibiotic resistant germs. The destroyed or broken proteins can be found in bacteria, but also, for example, in signaling molecules of the inflammation cascade such as TNF-alpha or interleukins. The antimicrobial silver ions can also block the signaling molecules resulting in an anti-inflammatory effect. This means that many skin problems caused by unwanted bacteria or inflammatory processes can be effectively addressed. Even for dry, cracked skin, MicroSilver BG™ creates an antimicrobial protective barrier on the skin.
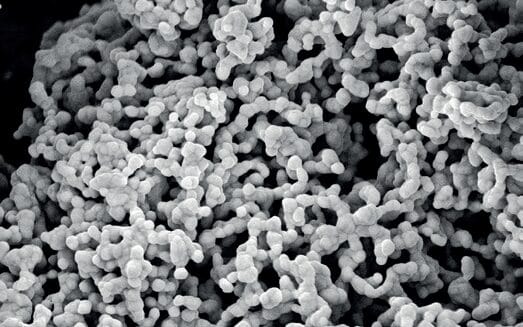
The skin microbiome is a complex system of microorganisms that live on and in the upper layers of the human skin. The bacteria of the resident flora are kept rejuvenated by regrowing bacteria from deeper layers of the skin. Therefore, cosmetic ingredients that do not interfere with the dynamics of recolonization of the skin microbiota are of high value. Combining such properties with antimicrobial attributes provides an even further enhancement. Such ingredients, like MicroSilver BG™, are a natural choice for microbiome-friendly skin care products with extended care properties. One of the key features of MicroSilver BG™ is its particle size and structure (Fig. 1). The particle size of 10 μm, on average, prevents penetration into deeper skin layers under the stratum corneum. A study already performed in 2007 (Institute Dr. Schrader and Austrian Research Centers GmbH 2007) using MicroSilver BG™ skin care products provided the first evidence of the microbiome-preserving property of MicroSilver BG™ as a cosmetic ingredient. Here it was shown that MicroSilver BG™ is not able to penetrate human skin and therefore it is not able to reach the “kindergarten” of the skin flora in the deeper layers of the skin. As a result, it cannot cause any damage to the bacteria located there, which continuously grow upwards to the top of the skin. This was also reported in later publications by Steinrücke in 2017a.
Highly porous particle:
Surface area: up to 5 m2/g
Silver Porosity: 90 – 95%
Average particle size: 10 μm
No nano particles
INCI Name: Silver
CAS No.: 7440-22-4
Approvals/Certifications:
FDA Masterfile
EPA (USA)
NPA
ECOCERT / COSMOS
Vegan
Microbiome studies
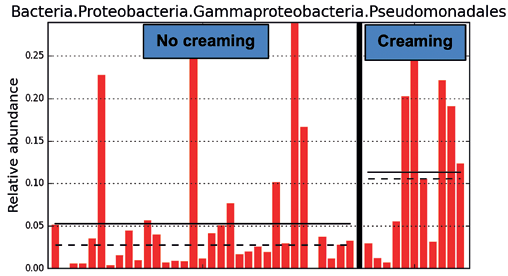
In a pre-study for the primary microbiome study, an anionic hydrophilic base cream (DAB basis cream) was used on 11 subjects with healthy skin. The cream was applied to one forearm during a 10-day continuous application program. The other forearm was left uncreamed. Microbiome analysis was performed before creaming and after 10 days. It was observed that creaming increased the relative abundance (Fig. 2) of germs particularly that of the Pseudomonadales signatures. Similar results were observed by Cui et al. in 2023 where creaming with cosmetics containing complex polysaccharides increased the relative abundance of germs in the microbiome.
MicroSilver BG and its effect on the skin microbiome
The primary microbiome study (10 days of daily application) using the DAB cream with and without MicroSilver BG™ confirmed that MicroSilver BG™ preserves the skin’s microbiota and is therefore microbiome friendly. At the same time, it was shown that unwanted transient germs were reduced. The skin’s own microbes were thus strengthened demonstrating that the MicroSilver BG™ provided a supportive function.
Study design
This study was conducted, comparing the application of a DAB cream (anionic hydrophilic base cream) with and without Micro4-Silver BG™ involving 13 female volunteers (20–45 years). One cream containing the addition of 0.1% MicroSilver BG™. The MicroSilver BG™ cream was applied on the left forearm and the base cream without MicroSilver BG™ on the right forearm during the 10 days.
Materials and methods:
- The sampling (BBl culture swabs, Becton Dickinson) was performed two times.
- The outer part of the forearm was swabbed with pre-moistened swabs (50mM Tris (pH 7.2), 1mM EDTA, 0.5% Tween20) about 50 times.
- Study subjects applied the MicroSilver BG™ containing cream for 10 days (left arm), and a control cream (without MicroSilver BG™; right arm) for 10 days.
- Control samples: Arm right, Arm left at t0; Arm right at t1 (10 days)
- MicroSilver BG™ samples: Arm left (with applied cream) at t1
- After sampling, swabs were immediately frozen at -80°C until DNA extraction.
- DNA extraction was performed using the XS-buffer method
- The concentration of the DNA was determined via Qubit and PCR was performed using 20 ng of DNA.
- Primers for PCR were used as published earlier (Caporaso 2011).
- Library construction and NGS sequencing was performed
using Illumina MiSeq - Raw reads were obtained after amplicon sequencing and processed Qiime2
- 16S rRNA gene biome table was analyzed using Calypso (http://cgenome.net/calypso/) and R
- For Calypso, the settings were as follows:
- TSS and square root normalization
- No samples removed; no taxa removed; no rare taxa removed. Included top 3000 taxa.
- Functional diversity was assessed using Picrust (Langille 2013).
- All data were statistically compared using Lefse (Segata 2011)
Germ identification and counting involved extraction of DNA from the collected skin samples, PCR, Amplicon sequencing, OTU-based 16S ribosomal subunit RNA analyses (Bjerre 2019, Caporaso 2011).
Results and quantitative visualizations
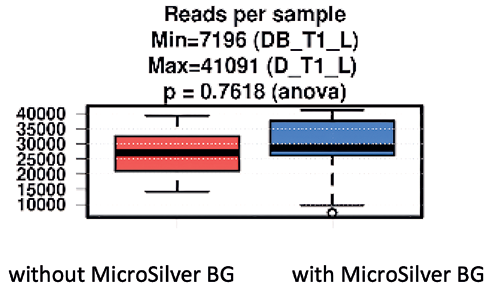
Major bioinformatics and statistical analysis are described below. Application of silver only slightly increased the number of detectable microbial signatures in a non-significant way (Fig. 3).
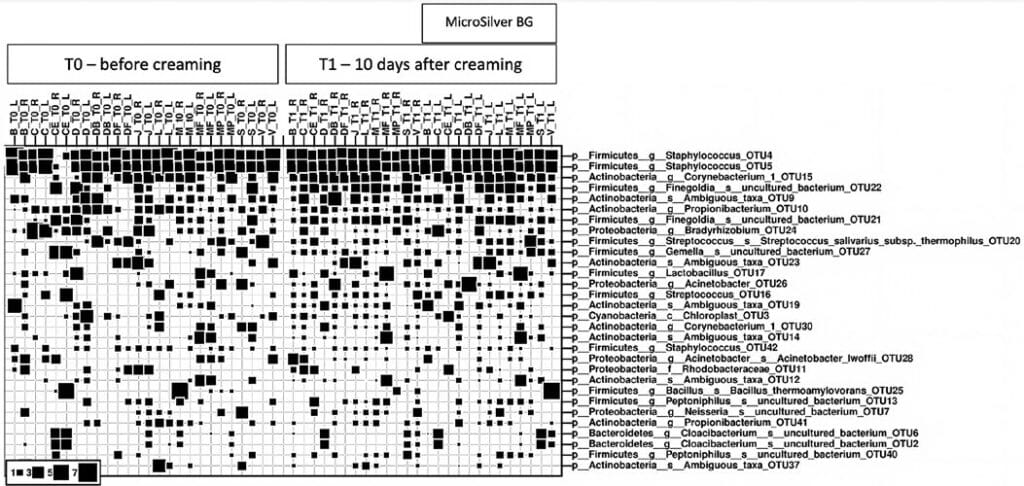
Further, the identification of the dominating microbial signatures in the strain level at both time points (0 and 10 days) are depicted below (Fig. 4). Bubble size reflects the relative abundance of certain microbial taxa. i.e. Staphylococcus OTU4/OTU5 was found to be most abundant amongst all samples, followed by Corynebacterium OTU15 and Finegoldia OTU22.
As expected, the skin microbiome is, amongst others, dominated by species signatures from Staphylococcus (OTU4, 5), Corynebacterium (OTU15) and Propionibacterium (= Cutibacterium; OTU 10) and thus reveals a typical microbiome profile.
After application (all samples on the right marked with “T1”), changes are not obvious, but a more homogenous picture is observed. This confirms our initial findings, that skin cream application per se has an impact on the microbiome composition.
The dominating microbial signatures (strain level) at timepoint 1 are depicted in the figure (Fig. 5). In this plot, the status of the microbial community (strain/species level) at the timepoint after application of the skin cream with/without MicroSilver BG (after 10 days) are compared. Next to the typical microbiome composition, the bubble plot does not reveal visible changes. Both arms, left and right, were treated with cream; the left arm was treated with cream containing MicroSilver BG™.
Bubble size reflects the relative abundance of certain microbial taxa. Staphylococcus OTU4/OTU5 were found to be most abundant amongst all samples, followed by Corynebacterium OTU15 and Finegoldia OTU22. Various strains of Staphylococcus are common skin microorganisms, as is also Corynebacterium and Propionibacterium. Finegoldia as well colonizes typically skin and also mucous membranes.
As expected, the skin microbiome is dominated by species signatures from Staphylococcus (OTU4, 5), Corynebacterium (OTU15) and Propionibacterium (= Cutibacterium; OTU 10). Also, Figures 4 and 5 show that the application of MicroSilver BG™ containing cream leads to a more homogenous microbiome profile.
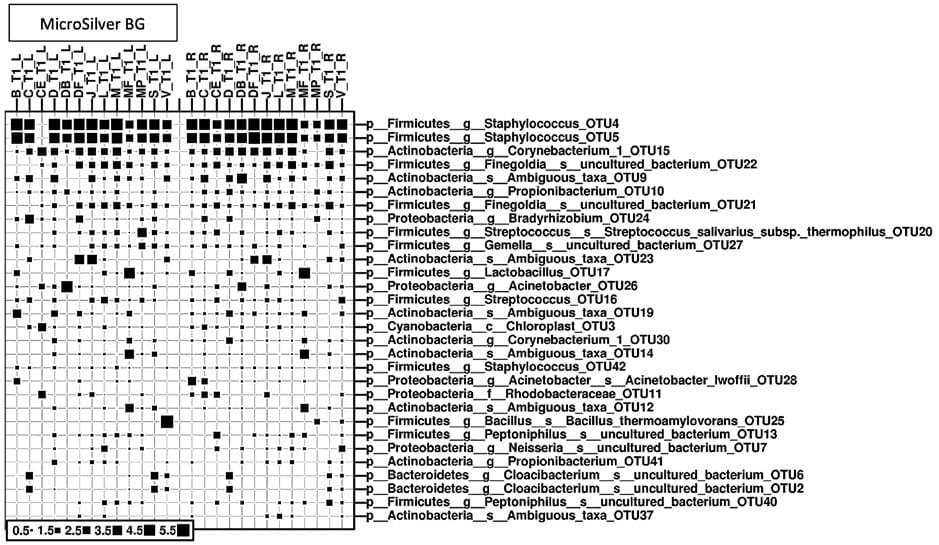
Does the application of MicroSilver BG™ impact the global microbiome profile?
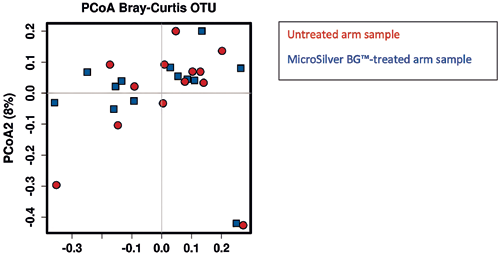
The following PCoA plot (Fig. 6) compares only timepoint 1 (after 10 days of treatment). The axes explain maximally 11% of the entire variance, which means the data interpretation has to be done with caution. However, no significant grouping of the MicroSilver BG™ samples is observed.
A very similar picture was retrieved with the outliers removed.
Adonis describes how variation in community composition can be attributed to different experimental treatments or control variables. Adonis describes if the community composition is different between groups.
Microbial community diversity as a result of the application of MicroSilver BG™ cream are depicted below (Fig. 7).
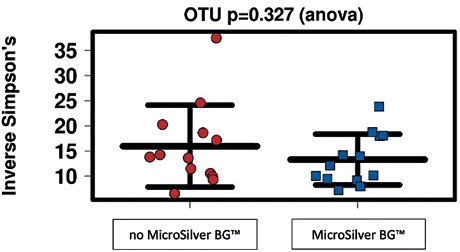
This plot compares the microbial community diversity in skin areas treated with and without MicroSilver BG™ at timepoint 1 (after application of skin creams). This parameter (inverse simpson index) indicates the richness in a community with uniform evenness that would have the same level of diversity. It is observed that the diversity in samples from MicroSilver BG™-treated skin is insignificantly affected.
What happens to germs of the transient skin flora?
The impact of silver application on signatures of certain microbial genera is followed by the LEfSE (Linear discriminant analysis effect size) method.
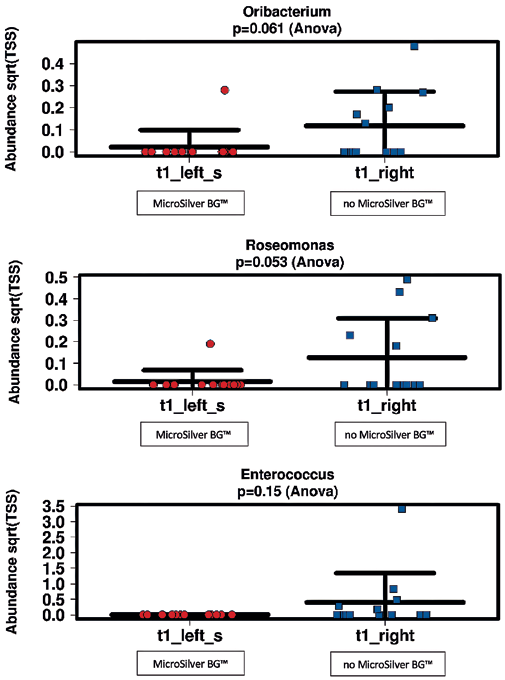
LEFSE listed as output, based on 300 most abundant OTUs (only timepoint 1: after 10 days) the following germs (LDA Score (log 10)):
- Oribacterium (LDA Score: 3.314, t1 right)
- Roseomonas (LDA Score: 3.373, t1 right)
- Enterococcus (LDA Score: 3.618, t1 right)
The silver treatment does affect the abundance (Fig. 8) of signatures of Oribacterium, Roseomonas and Enterococcus. However, all these genera are rather low abundant (0.04% of all reads:
Oribacterium; 0.1% of all reads: Roseomonas; 0.28% of all reads: Enterococcus) on skin.
Obviously, the abundance of certain unfavourable transient germs (genus-signatures) is affected by MicroSilver BG application. Roseomonas and Enterococcus signatures were found to be lowered under MicroSilver BG treatment.
Roseomonas is considered as an opportunistic bacterium of the human microbiome which is sometimes seen in primary or healthcare associated infections (Romano-Bertrand 2016).
Enteroccus bacteria are well-known germs of nosocomial infections (Said 2022). Oribacterium is an anaerobe, mesophilic, Gram-positive human pathogen that can be found in the sinus maxillaris (Sizova 2014). It is considered to be a transient germ on the skin.
Conclusion
The study confirmed that skincare products with MicroSilver BG™ as a skin conditioning agent with antimicrobial properties do not change the composition of the natural healthy microbiome. It was shown that some unfavorable transient germs are reduced in relative abundance. Data analysis confirmed that skincare with MicroSilver BG™ keeps the natural resident skin flora more homogenous and in good balance. Slightly more reads were obtained in the samples treated with MicroSilver BG™ cream indicating slightly increased microbiome signatures. MicroSilver BG™ treatment and creaming homogenized the microbial profile amongst the subjects.
Thanks to its porous coral-like structure MicroSilver BG™ resides on top of the skin and also adheres very well which provides better and prolonged leave-on properties. So, this placement and long-lasting effect is at an ideal location where it can remove transient germs on the skin surface. The natural skin flora and the natural acid mantle it maintains are left intact. This resulted in a conserved evenly spread microbiota.
The compelling observation is that MicroSilver BG™ is a “microbiome-friendly” skin conditioner with antimicrobial properties that helps stressed skin to rebalance and stabilize itself.
MicroSilver BG™ is an effective ingredient for modern microbiome supportive skincare products.
References
- Lusk BG: Thermophiles; or, the Modern Prometheus: The Importance of Extreme Microorganisms for Understanding and Applying Extracellular Electron Transfer. Front. Microbiol. 10: 818. (2019)
- Price PB: The bacteriology of normal skin: a new quantitative test applied to a study of bacterial flora and disinfectant action of mechanical cleansing. J Infect Dis 1938; 63: 301–318.
- Elsner P: What textile engineers should know about the human skin. Curr Probl Dermatol. 2003; 31: 24–34
- Hendley JO, Ashe KM: Effect of topical antimicrobial treatment on aerobic bacteria in the stratum corneum of human skin. Antimicrob Agents Chemother. 1991; 35: 627–631. 8.
- Marples MJ: The normal microbial flora of the skin. Society for applied bacteriology symposium series. 1974; 3(0): 7–12.
- Costello EK et al.: Bacterial Community Variation in Human Body Habitats Across Space and Time. Science. 2009 December 18; 326(5960): 1694–1697
- De Pessemier B, Grine L, Debaere M, Maes A, Paetzold B, Callewaert C. Gut-Skin Axis: Current Knowledge of the Interrelationship between Microbial Dysbiosis and Skin Conditions. Microorganisms. 2021 Feb 11;9(2):353. doi: 10.3390/microorganisms9020353. PMID: 33670115; PMCID: PMC7916842.
- Ekanayake-Mudiyanselage, S., Balk, A., Schoder, V., Hansen, P., Wigger-Alberti, W., & Wilhelm, K., Use test to evaluate the efficacy, tolerability and cosmetic acdeptance of a new topical silver containing care product (Multilind MikroSilber Creme) in atopic dermatitis, Kosmetische Medizin 6/2007:291-295
- Müller-Steinmann J., Golbach U., Höhn S., Langenauer M., Prospective dermatologically controlled study of the efficacy of a silver containing nurturing cream (MicroSilver BG™ 0.1%) in atopic dermatitis, Kosmetische Medizin 01/2008, 29(4):208-212
- Neub A., Amon U., Adjuvant skin care with a Micro-Silver containing emollient with DMS-lipid-structure: efficacy and cosmetical acceptance in atopic eczema and psoriasis, Kosmetische Medizin 2/2009: 20–24
- Müller-Steinmann J., Mikro-Silber-Präparat unterstützt Aknetherapie effizient (Micro-silver preparation efficiently supports acne therapy). Ästhetische Dermatologie 2/2010: 3–5
- Langenauer M., Microsilver – An Active Ingredient for Skin and Oral Care Applications. SOFW Journal 2011, 5: 54–57
- Golbach U., Müller-Steinmann J., Höhn S., Prospective dermatologically controlled efficacy study of a hydrating spray emulsion with 0.2% elemental silver in atopic dermatitis, Kosmetische Medizin 03/2011: 129–135
- Barbareschi M., Alessandrini G., Cardinali C., Gimma A., Veraldi S., Efficacy and tolerability of a novel topical agent in mild acne: results of a prospective multicenter study, European Journal of Acne and Related Diseases, Vol. 6, n. 3, 2015: 77–81
- Steinrücke P., Porous Microparticulate Silver: Non-Penetrating, Agglomerated Topical, Antibacterial & Anti-Inflammatory Active for Acne Skin Care, Euro Cosmetics, Volume 25, 3-2017, 12–16
- Steinrücke P., Rosacea: The curse of the celts – A porous silver lining for an efficient rosacea skincare, Euro Cosmetics, Volume 25, 11/12-2017, 2–6
- Institute Dr. Schrader, Testreport from 08. April 2021, 357-HP-07-063-07-238 / BE_HP-07-162_01 Tape-Strippig (Silver-Penetration-Study) and Determination of the silver content in sellotapes after application of a silver containing ointment on human skin, Austrian Research Centers GmbH, 2007 (unpublished)
- Cui S, Pan M, Tang X, Liu G, Mao B, Zhao J, Yang K. Metagenomic insights into the effects of cosmetics containing complex polysaccharides on the composition of skin microbiota in females. Front Cell Infect Microbiol. 2023 Aug 1;13:1210724. doi: 10.3389/fcimb.2023.1210724. PMID: 37593763; PMCID: PMC10428012.
- Caporaso JG, Lauber CL, Costello EK, Berg-Lyons D, Gonzalez A, Stombaugh J, Knights D, Gajer P, Ravel J, Fierer N, Gordon JI, Knight R. Moving pictures of the human microbiome. Genome Biol. 2011;12(5):R50. doi: 10.1186/gb-2011-12-5-r50. PMID: 21624126; PMCID: PMC3271711.
- Langille MG, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA, Clemente JC, Burkepile DE, Vega Thurber RL, Knight R, Beiko RG, Huttenhower C. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol. 2013 Sep;31(9):814-21. doi: 10.1038/nbt.2676. Epub 2013 Aug 25. PMID: 23975157; PMCID: PMC3819121.
- Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011 Jun 24;12(6):R60. doi: 10.1186/gb-2011-12-6-r60. PMID: 21702898; PMCID: PMC3218848.
- Bjerre RD, Hugerth LW, Boulund F, Seifert M, Johansen JD, Engstrand L. Effects of sampling strategy and DNA extraction on human skin microbiome investigations. Sci Rep. 2019 Nov 21;9(1):17287. doi: 10.1038/s41598-019-53599-z. PMID: 31754146; PMCID: PMC6872721.
- Romano-Bertrand S, Bourdier A, Aujoulat F, Michon AL, Masnou A, Parer S, Marchandin H, Jumas-Bilak E. Skin microbiota is the main reservoir of Roseomonas mucosa, an emerging opportunistic pathogen so far assumed to be environmental. Clin Microbiol Infect. 2016 Aug;22(8):737.e1-7. doi: 10.1016/j.cmi.2016.05.024. Epub 2016 Jun 3. PMID: 27269884.
- Said MS, Tirthani E, Lesho E. Enterococcus Infections. 2022 May 2. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan–. PMID: 33620836.
- Sizova MV, Muller PA, Stancyk D, Panikov NS, Mandalakis M, Hazen A, Hohmann T, Doerfert SN, Fowle W, Earl AM, Nelson KE, Epstein SS. Oribacterium parvum sp. nov. and Oribacterium asaccharolyticum sp. nov., obligately anaerobic bacteria from the human oral cavity, and emended description of the genus Oribacterium. Int J Syst Evol Microbiol. 2014 Aug;64(Pt 8):2642-2649. doi: 10.1099/ijs.0.060988-0. Epub 2014 May 13. PMID: 24824639; PMCID: PMC4129163.