
Summary
The marketing services of cosmetic manufacturers highly value exceptional imagery, recognizing the persuasive impact of creatively visualising the effects of their products. Such exceptional imaging serves as a vital foundation for a successful advertising campaign and commercial success. Here, we aim to outline cutting-edge methods for conducting cosmetic efficacy tests, enabling the generation of three-dimensional images and animations using new generation state-of-the-art technology. Recent advancements in microscopy, coupled with progress in skin tissue clearing techniques, as well as X-ray microtomography, with line-field confocal optical coherence tomography, now enable the creation of 3D imaging and animations. Presenting efficacy test results for cosmetic products through high-quality 3D photographs and videos represents a new and compelling reality within the industry.
Introduction
The primary goal of any marketing endeavour is the commercial success of a product. A key factor in achieving this success is the infusion of creativity into arguments, which significantly aids in persuading customers that a particular product is not only unique but also efficient, making it a must-have item. In the realm of cosmetics, European regulations explicitly dictate that the efficacy of such products must be substantiated1. To assess the biological effects of cosmetics, clinical tests and studies on living human skin explants ex vivo are widely employed.
While studies involving volunteers provide real-world conditions of product use, they are constrained by a limited range of analytical tools, encompassing non-invasive instrumental measurements and skin appearance imaging. On the other hand, the ex vivo model enables the evaluation of a product’s effects within various skin layers, elucidating the mechanism of action by visualising target proteins. Traditional approaches involve histological examination of skin morphology, coupled with specific immunostaining of thin sections. However, despite 400 years since the invention of the microscope by Van Leeuwenhoek, histology has predominantly yielded two-dimensional (2D) images. Although there exists the capability to reconstruct three-dimensional (3D) skin structures from imaging serial sections, its application remains restricted due to its time-consuming, inaccurate, and error-prone nature2.
3D imaging on skin explants ex vivo
X-ray microtomography enable exploration of the skins internal microstructure. Utilizing X-rays, this method generates optical cross-sections of a human skin explant, as the sample rotates
around its axis positioned between the source of rays and the detector. Series of 2D projections with pixels in the micrometer range are used to reconstruct a virtual 3D-model of a skin sample
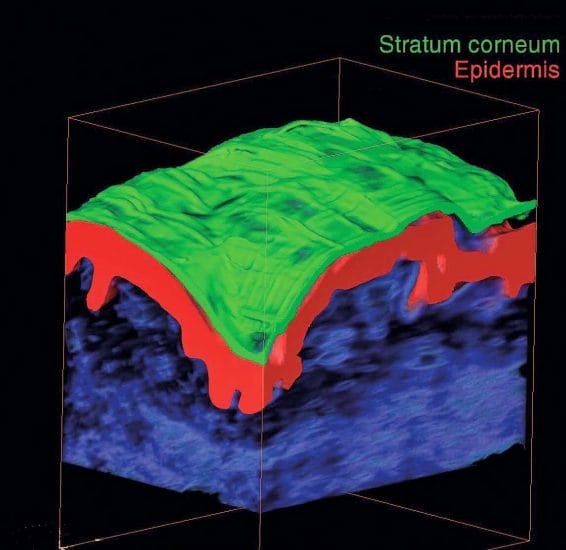
without destroying it. High resolution, fast acquisition and nondestruction are major advantages of this technique 3. In efficacy studies for cosmetic products, it can be used for 3D characterization of the dermo-epidermal junction, orientation of dermal fibers, measurement of thickness of skin layers and analysis of sebaceous glands (Figure 1).

Recent progress in two-photon confocal laser scanning microscopy and light sheet microscopy made 3D imaging of tissues possible. The confocal microscope illuminates only one point in the
sample at a time, filtering out-of-focus light. Image is constructed point by point, moving deeper in the tissue. It is a long process with high risk of photobleaching 4. When illumination of a thin
plane of the sample takes place from the side perpendicular to angle of observation, a light-sheet microscope produces images of the entire sheet. This means the skin is sectioned optically. It is faster and less photo-toxic comparing to confocal microscopy Stacks of serial images are merged to reconstruct the 3D spatial architecture of skin 6.
However, skin opaqueness seriously limits proper accurate imaging, since the skin is non-transparent, because light is not able to pass in a straight line through the tissue. Light scatters when it reaches cell membranes or collagen fibers in the dermis because they have a different refractive index 7. Furthermore, melanin absorbs light, reducing image quality further, making them blurry and dim 8.
More than a century ago a first method to make tissue transparent was presented by Spalteholz9. Using optical clearing agents, it is possible to decrease the heterogeneity of refractive indices in the skin, reducing light scattering and absorbance, therefore, making the skin more transparent. At present, ten optical tissue clearing (OTC) approaches are used for imaging of ex vivo skin explants 10. They can be roughly split into three groups: organicsolvent
based clearing, aqueous clearing, and the CLARITY-based method.
The concept of the CLARITY approach is to replace lipids by a polymerised acrylamide forming artificial skeleton 11. It successfully clears brain, lung or liver tissues and permits immunostaining afterwards. However, it is time-consuming, technically difficult, and less adapted for skin clearing 12. On the other hand, Aqueous clearing is based on lipid removal with detergents followed by intensive hydration with urea or formamide. These methods are simple and provide good preservation of fluorescence signals. They are efficient only for tissues with little extracellular matrix (brain, heart, kidney) 14, 15.
In the organic solvent-based procedure tissue should firstly be dehydrated before clearing. It is an excellent choice for skin clearing – efficient, fast and user-friendly (Figure 2). However, a researcher should be careful as regards fluorescence quenching and tissue shrinkage.


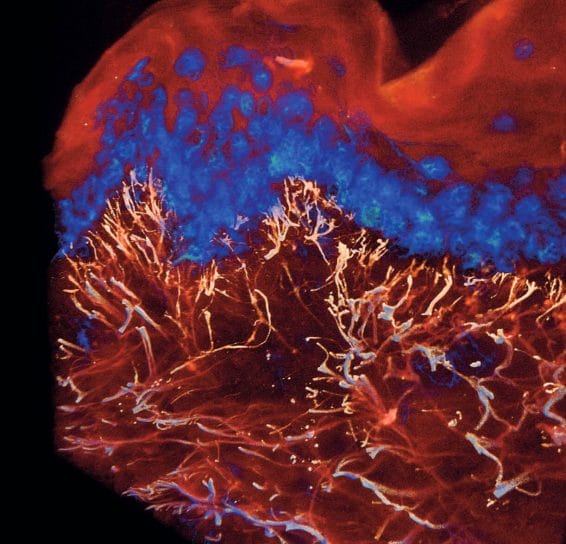
Efficient optical skin clearing, when coupled with state-of-theart confocal or light-sheet microscopy, enables the acquisition of stunning 3D images of human skin. Even the basic imaging of auto-fluorescent signals from the skin yields captivating 3D pictures (see Figure 3).
By employing targeted antibodies against specific proteins, one can readily emphasise the purported effects of cosmetics within tissues (see Figure 4). This heralds a novel era in the presentation of data derived from efficacy studies of cosmetic products. Utilising 3D imaging and video proves to be invaluable for bolstering the marketing efforts supporting cosmetic claims. These narrative-driven visualisations play a crucial role in persuading the public of the efficacy and exclusivity of the products.
In vivo tools for non-invasive 3D imaging
The remarkable advancements achieved in rendering fixed ex vivo skin transparent have spurred researchers to push the boundaries and delve into the development of innovative approaches for studying dynamic processes within living tissues. The evolution of in vivo optical clearing techniques has become a pivotal milestone in this pursuit. Various methods, such as laser irradiation, stripping, micro-needling, and micro-ablation, have been instrumental in facilitating the penetration of clearing agents into living tissues17, 18, 19.
The synergy of in vivo optical clearing with state-of-the-art optical imaging technologies has paved the way for obtaining superior parameters that are crucial for generating high-quality images. These parameters include enhanced contrast, resolution, sensitivity, and an increased depth of detection. Such advancements are paramount in unraveling intricate details within living tissues, providing researchers with a more comprehensive understanding of biological processes.
The application of in vivo clearing techniques has proven to be particularly successful in the detection of cutaneous dysfunction20. It has also demonstrated efficacy in the meticulous study of skin lesions21, 22. Furthermore, the integration of in vivo optical clearing has extended its impact to therapeutic realms, notably in the enhancement of light-induced therapies23. Beyond therapeutic applications, in vivo clearing has even found a role in cosmetic procedures, such as tattoo removal24.
The versatility of in vivo optical clearing not only broadens its applicability in diverse scientific domains but also underscores its potential for revolutionising medical diagnostics and interventions. As researchers continue to refine and expand these techniques, the future holds exciting possibilities for gaining unprecedented insights into the dynamic processes occurring within living tissues.
However, the realm of imaging devices extends beyond the purview of ex vivo 3D reconstructions, venturing into a diverse array of cutting-edge techniques designed for real-time visualisation and assessment of dynamic biological processes within living human skin tissue. Among these avant-garde methodologies are Laser Speckle Contrast Imaging (LSCI), Hyperspectral Imaging (HSI), Optical Coherent Tomography (OCT), Photoacoustic Imaging (PAI), Surface-Enhanced Raman Scattering Imaging, Flow Cytometry, and Laser Confocal Microscopy25.
While these techniques offer remarkable insights into the intricacies of living tissue, the application of in vivo optical clearing in the cosmetic field, remains largely unexplored. Presently, there is a notable absence of its utilisation, marking a gap in the potential enhancement of cosmetic procedures. The challenge at hand lies in the development of methods that not only ensure the safety and efficacy of in vivo optical clearing in human skin but also harmonise seamlessly with economically viable imaging techniques. The convergence of these elements represents a significant and ongoing challenge in the pursuit of advancing our understanding and capabilities in the cosmetic domain. The establishment of reliable and accessible approaches to in vivo optical clearing coupled with affordable imaging technologies stands as a paramount objective, holding the key to transformative breakthroughs in the field. As researchers continue to delve into this uncharted territory, the promise of refined, real-time visualisation techniques for dynamic processes within living human skin remains a tantalising prospect on the horizon.
Being medical devices in origin, LC-OCT and Laser scanning microscopy techniques are used more and more often in clinical studies for “anti-ageing” cosmetics. They provide a non-invasive in-depth 3D imaging of human skin in vivo with possibilities to analyze epidermal thickness, relief of the dermo-epidermal junction, and dermis density. (Figure 5)26
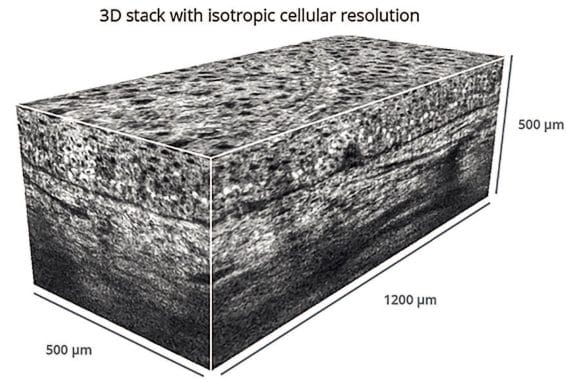
These techniques are characterized by a good ratio between the resolution of images and depth of view. Evolution of skin responses upon product treatment from different body areas can be visualized and analyzed. Currently, their popularity is still limited by relatively high cost of image acquisition and data interpretation.
Concluding Remarks
Various techniques are already available to obtain 3D images from cosmetic studies ex vivo and in vivo, and the technical progress in microscopy provides the cosmetic industry with innovative types of imaging. This is a new level of visualization of the skins appearance in response to cosmetic application, and optical skin clearing is one of the most interesting and dynamic approaches. The fundamental concept behind optical tissue clearing involves utilising chemical substances to standardise all refractive indexes. This process reduces light scattering, rendering the tissue transparent and eliminating the need for histological sections. Consequently, specific microscopy enables the easy visualisation of deep skin structures. By capturing a stack of 2D images at various depths and merging them, a 3D reconstruction of the skin’s spatial architecture can be generated. The application of a cosmetic product on ex vivo skin reveals its biological effects throughout the entire analysed tissue volume. This collaboration between physicians, chemists, and biologists has provided a groundbreaking tool for marketing specialists. It exemplifies a non-linear cooperation, where fundamental scientific research, supported by applied science specialists, culminates in the development of a hydrating cream.
Acknowledgements
The author thanks CCI Hamburg, Germany for their editorial and suggestions, Imactiv-3D SAS, Toulouse, France, Novitom, Grenoble, France and Damae Medical, Paris, France for providing the 3D images.
References
- REGULATION (EC) No 1223/2009 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 30 November 2009 on cosmetic products. Official Journal of the European Union. L 342/59 – 209. Link: http://data.europa.eu/eli/reg/2009/1223/oj
- Griffini, P. et al. Three-dimensional reconstruction of colon carcinoma metastases in liver. J. Microsc. 1997; 187 (Pt 1), 12–21. DOI: 10.1046/j.1365-2818.1997.2140770.x
- Walsh, C.L. et al. Imaging intact human organs with local resolution of cellular structures using hierarchical phase-contrast tomography. Nat Methods 2021; 18: 1532–1541. DOI https://doi.org/10.1038/s41592-021-01317-x
- Marx V. Microscopy: seeing through tissue. Nat Methods 2014; 11:1209-14. DOI: https://doi.org/10.1038/nmeth.3181
- Zhu D. et al. Recent progress in tissue optical clearing. Laser Photonics Rev. 2013; 7: 72-57. DOI: 10.1002/lpor.201200056
- Keller PJ, Ahrens MB. Visualizing whole-brain activity and development at the single-cell level using light-sheet microscopy. Neuron 2015;85: 462-83. DOI: 10.1016/j.neuron.2014.12.039.
- Tuchin VV. Tissue optics and photonics: light-tissue interaction. J Biomed Photonics Amp Eng 2015b; 1: 98-134. DOI: https://doi.org/10.18287/jbpe-2015-1-2-98
- Tan Y. et al. Research techniques made simple : Optical clearing and Three-dimensional volumetric imaging of skin biopsies. J Invest. Dermat. 2020; 140: 1305—1314. DOI: https://doi.org/10.1016/j.jid.2020.04.014
- Spalteholz, W., 1911. Über das Durchsichtigmachen von Menchlichen und Tierichen Präparaten und Seine Theoretischen Bedingungen. S. Hirzel, Leipzig.
- Azaripour, A., et al., A survey of clearing techniques for 3D imaging of tissues with special reference to connective tissue. Prog Histochem Cytochem (2016), http://dx.doi.org/10.1016/j.proghi.2016.04.001
- Chung, K. et al Structural and molecular interrogation of intact biological systems. Nature 2013; 497 (7449), 332–337. DOI: https://doi.org/10.1038/nature12107
- Tomer, R. et al. Advanced CLARITY for rapid and high-resolution imaging of intact tissues. Nat. Protoc. 2014; 9 (7), 1682–1697. DOI: https://doi.org/10.1038/nprot.2014.123
- Susaki EA. at al. Advanced CUBIC protocols for whole-brain and whole-body clearing and imaging. Nat Protoc. 2015; 15: 1709-27. DOI: https://doi.org/10.1038/nprot.2015.085
- Tainaka K. et al. Whole-body imaging with single-cell resolution by tissue decolorization. Cell 2014; 159: 911-24. DOI: 10.1016/j.cell.2014.10.034.
- Hama H. et al. ScaleS: an optical clearing palette for biological imaging. Nat Neurosci 2015; 18: 1518-29. DOI: 10.1038/nn.4107
- Kuck M et al. In vivo enhancement of imaging depth for optical coherence tomography by eudermic agents on ridged and meshed human skin. Laser Phys. Lett. 2014; 11(3). DOI: 10.1088/1612-2011/11/3/035602
- Genina E.A. et al. Integrated effects of fractional laser microablation and sonophoresis on skin immersion optical clearing in vivo. J. Biophotonics 2020; 13(7). DOI: 10.1002/jbio.202000101
- Damestani Y et al. Optical clearing agent perfusion enhancement vis combination of microneedle poration, heating and pneumatic pressure. Lasers Surg. Med. 2014; 46(6): 488-498. DOI: 10.1002/lsm.22258
- Pires L et al. Optical clearing of melanoma in vivo: characterization by diffuse reflectance microscopy and optical coherence tomography. K. Biomed Opt. 2016; 21(8). DOI: 10.1117/1.JBO.21.8.081210.
- Feng W. et al. Quantitative evaluation of skin disorders in type I diabetic mice by in vivo optical imaging. Biomed Opt Express 2019; 10(6): 2996-3008. DOI: 10.1364/BOE.10.002996
- Pan J. et al. A miniaturized fluorescence imaging device for rapid early skin cancer detection. J. Innov. Opt. Health Sci. 2021; 14(2). DOI: https://doi.org/10.1142/S1793545820500261
- Liu Y. et al. Penetration-enhanced optical coherence tomography with optical clearing agent for clinical evaluation of human skin. Photodiagn. Photodyn. 2020; 30. DOI: 10.1016/j.pdpdt.2020.101734.
- Genin V.D. et al. Influence of immersion agents on optical parameters of bio-tissues during laser photothermal therapy of tumor: pilot study. Opt. Spectrosc. 2022: 130(6): 678-687. DOI: 10.21883/EOS.2022.06.54704.27-22
- Liu C. et al. Quantitative evaluation of enhanced laser tattoo removal by skin optical clearing. J. Innov. Opt. Health Sci. 2015; 8(3). DOI: https://doi.org/10.1142/S1793545815410072
- Xia Q et al. In vivo skin optical clearing for improving imaging and light induced therapy: a review. J. Biomed. Opt. 2023; 28(6). DOI: 10.1117/1.JBO.28.6.060901
- Ayadh M. et al. LC-OCT imaging for studying the variation of morphological properties of human skin in vivo according to age and body area: the forearm and thigh. Dermis 2022; 2(1): 2. DOI: 10.35702/Derm.10002



